Robert Boyle's Electrolysis Of Water Showed That _____.
umccalltoaction
Nov 16, 2025 · 10 min read
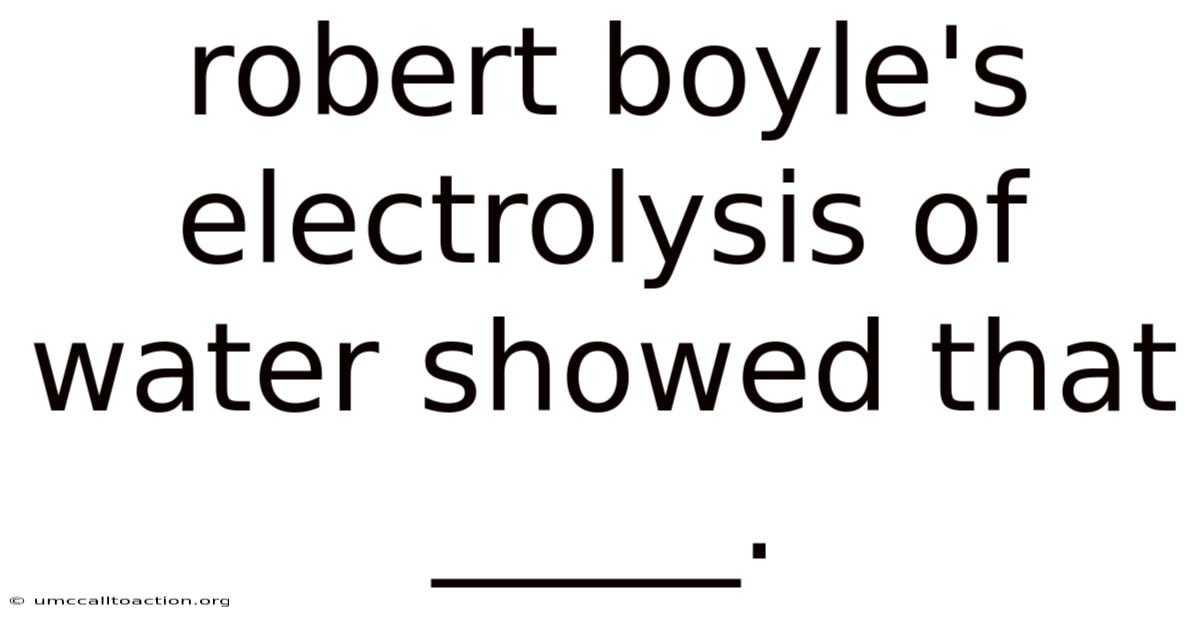
Table of Contents
Robert Boyle's electrolysis of water experiment revealed a groundbreaking truth about the composition of this fundamental substance, forever changing our understanding of matter and laying the foundation for modern chemistry.
The Genesis of Scientific Inquiry: Robert Boyle and His Era
Robert Boyle, a towering figure of the 17th century, stands as one of the founding fathers of modern chemistry. Born into an era steeped in alchemy and Aristotelian philosophy, Boyle championed a new approach to understanding the natural world: empirical observation and experimentation. His work marked a significant departure from speculative theories, emphasizing the importance of evidence-based reasoning. Boyle believed that by meticulously observing and experimenting with substances, one could unlock the secrets of their composition and behavior. He tirelessly advocated for controlled experiments, accurate measurements, and the rejection of unsubstantiated claims, principles that remain cornerstones of scientific methodology today.
Boyle's contributions extended beyond the laboratory. He was a prolific writer, publishing numerous works that disseminated his findings and promoted his scientific philosophy. His most famous book, The Sceptical Chymist, published in 1661, is considered a seminal text in the history of chemistry. In it, Boyle challenged the prevailing alchemical and Aristotelian views on the elements, arguing for a more rigorous definition based on experimental evidence. He proposed that matter was composed of indivisible particles, a concept that foreshadowed the atomic theory of matter. While Boyle didn't fully develop the atomic theory, his work laid the groundwork for future scientists like John Dalton to build upon.
Moreover, Boyle was deeply interested in the practical applications of science. He conducted experiments on a wide range of topics, including gases, combustion, respiration, and the properties of acids and bases. He invented the air pump, which allowed him to study the behavior of gases under different pressures, leading to the formulation of Boyle's Law, which states that the volume of a gas is inversely proportional to its pressure at a constant temperature. This law, a fundamental principle in physics and chemistry, demonstrated the power of quantitative experimentation in understanding the natural world. Boyle's legacy extends far beyond his individual discoveries. He played a crucial role in establishing chemistry as a distinct scientific discipline, separate from alchemy and medicine. His emphasis on empirical observation, experimentation, and logical reasoning helped to shape the scientific method and laid the foundation for the modern scientific enterprise.
Unveiling Water's Secrets: The Context of Boyle's Electrolysis Experiment
Prior to Boyle's experiments, water was widely regarded as a fundamental element, an indivisible substance that could not be broken down into simpler components. This view, rooted in ancient Greek philosophy, had persisted for centuries, shaping scientific thought and hindering the development of a true understanding of water's composition. However, a growing body of evidence began to challenge this long-held belief. Alchemists, in their quest to transmute base metals into gold, had observed various reactions involving water, noting its ability to dissolve substances and participate in chemical transformations. These observations hinted at the possibility that water might not be as simple as it seemed.
The prevailing understanding of matter during Boyle's time was largely based on the Aristotelian concept of four elements: earth, air, fire, and water. These elements were believed to be the fundamental building blocks of all substances, with different combinations of these elements giving rise to the diverse range of materials found in the world. Alchemy, a blend of philosophy, mysticism, and practical experimentation, sought to understand the properties of matter and manipulate it to achieve specific goals, such as the transmutation of metals and the creation of elixirs of life. Alchemists conducted numerous experiments involving heating, distillation, and mixing of substances, often observing unexpected and unexplained phenomena. These observations, while not always interpreted correctly, provided valuable clues about the nature of matter and paved the way for future scientific discoveries.
Boyle, however, approached the study of matter with a more critical and empirical mindset. He questioned the validity of the four-element theory, arguing that it was not supported by experimental evidence. He emphasized the importance of conducting controlled experiments and carefully observing the results, rejecting speculative theories that were not based on empirical data. Boyle's skepticism and his commitment to experimental observation led him to investigate the composition of various substances, including water. He sought to determine whether water was truly an indivisible element or whether it could be broken down into simpler components. This quest led him to conduct his famous electrolysis experiment, which would ultimately revolutionize our understanding of water and its fundamental nature.
The Experiment: Deconstructing Water Through Electrolysis
Robert Boyle's investigation into the composition of water involved a meticulous and groundbreaking experiment, one that would challenge the established scientific dogma of his time. While not exactly the electrolysis we know today (as electricity was not yet harnessed in the same way), Boyle's experiments involved heating water with iron filings. He observed that during this process, a gas was released, and the iron filings were transformed.
The core of Boyle's experimental setup involved carefully heating water in a sealed vessel, ensuring that any gases produced could be collected and analyzed. He introduced iron filings into the water, a key component of the experiment that would facilitate the decomposition of water. As the mixture was heated, Boyle meticulously observed the changes that occurred. He noted the formation of a gas, which he carefully collected and characterized. He also observed that the iron filings underwent a transformation, changing in appearance and properties.
Boyle paid close attention to the quantitative aspects of his experiment, carefully measuring the amount of water used, the amount of iron filings added, and the volume of gas produced. These measurements were crucial for drawing accurate conclusions about the composition of water and the nature of the chemical reaction that had taken place. By carefully analyzing the gas produced during the experiment, Boyle was able to determine some of its properties. He observed that it was flammable and that it supported combustion, suggesting that it was different from ordinary air. He also noted that the iron filings, after the experiment, had increased in weight, indicating that they had combined with something from the water.
Through careful observation and analysis, Boyle concluded that the gas produced during the experiment was not simply air that had been released from the water. Instead, he proposed that the gas was a component of water itself, which had been liberated by the reaction with iron. He reasoned that the iron filings had reacted with the water, extracting one of its components and forming a new substance. This conclusion was a revolutionary departure from the prevailing view that water was an indivisible element. Boyle's experiment provided strong evidence that water was, in fact, a compound, composed of at least two different substances.
The Revelation: Water is Not an Element
Robert Boyle's experiments, particularly his work involving iron filings and heated water, yielded a profound conclusion: water is not an indivisible element, but rather a compound comprised of simpler substances. This discovery challenged the widely accepted Aristotelian view that had dominated scientific thought for centuries, marking a pivotal moment in the history of chemistry. By demonstrating that water could be broken down into different components, Boyle shattered the notion of water as a fundamental element and paved the way for a more accurate understanding of its composition.
The significance of Boyle's findings extended far beyond the specific case of water. It provided a powerful demonstration of the limitations of the prevailing scientific theories and the importance of empirical observation and experimentation in uncovering the true nature of matter. Boyle's work inspired other scientists to question established beliefs and to conduct their own experiments to investigate the composition of other substances. This led to a period of rapid advancement in chemistry, as new elements were discovered and new compounds were synthesized.
Boyle's conclusion that water is a compound had a profound impact on the development of the atomic theory of matter. His work suggested that all substances, including water, were composed of smaller, indivisible particles, which he called corpuscles. While Boyle's concept of corpuscles was not exactly the same as the modern concept of atoms, it was a crucial step towards the development of the atomic theory. His ideas influenced later scientists like John Dalton, who built upon Boyle's work to formulate the first coherent atomic theory, which revolutionized our understanding of matter and its properties.
Furthermore, Boyle's discovery had significant implications for various fields of science and technology. It laid the foundation for the development of new methods for purifying water, for producing new materials, and for understanding chemical reactions. It also opened up new avenues of research in fields such as medicine, agriculture, and engineering. Boyle's legacy as a pioneering scientist and a champion of empirical observation continues to inspire scientists today. His work serves as a reminder of the power of experimentation in uncovering the secrets of the natural world and in advancing our understanding of the universe.
Connecting the Dots: Boyle's Legacy and Modern Chemistry
Robert Boyle's electrolysis of water experiment, though rudimentary by modern standards, holds immense historical significance. It demonstrated that water could be broken down into other substances, challenging the ancient Greek concept of water as a fundamental element. While Boyle did not fully understand the nature of the gases produced (hydrogen and oxygen), his experiment provided crucial evidence that water was not a simple substance, but a compound.
His work directly influenced later scientists, including Antoine Lavoisier, who identified oxygen and hydrogen as distinct elements. Lavoisier's experiments, building upon Boyle's foundation, definitively proved that water was composed of these two elements. This discovery revolutionized chemistry, providing a new framework for understanding chemical reactions and the composition of matter.
Boyle's emphasis on empirical evidence and quantitative measurement laid the groundwork for the scientific method. His insistence on rigorous experimentation and his rejection of unsubstantiated claims helped to transform alchemy into a true science. His legacy continues to inspire scientists today, reminding us of the importance of observation, experimentation, and critical thinking in the pursuit of knowledge.
FAQ: Delving Deeper into Boyle's Experiment
Q: Did Robert Boyle actually perform electrolysis in the modern sense?
A: Not exactly. Modern electrolysis uses electricity to split water molecules. Boyle's method involved heating water with iron filings, which resulted in a chemical reaction that released a gas.
Q: What was Boyle's understanding of the gas produced?
A: Boyle didn't fully understand the composition of the gas. He knew it was flammable and supported combustion, but he didn't identify it as hydrogen.
Q: Why is Boyle's experiment so important?
A: It challenged the long-held belief that water was an indivisible element and paved the way for a more accurate understanding of its composition.
Q: How did Boyle's work influence later scientists?
A: His emphasis on empirical evidence and experimentation inspired scientists like Lavoisier to further investigate the composition of water and other substances.
Q: What were the limitations of Boyle's experiment?
A: Boyle lacked the technology and knowledge to fully characterize the gases produced. He also didn't have a clear understanding of the chemical reactions involved.
Conclusion: The Enduring Impact of a Skeptical Chymist
Robert Boyle's electrolysis of water experiment, though seemingly simple, represents a pivotal moment in the history of science. It demonstrated the power of empirical observation and experimentation in challenging established beliefs and uncovering the true nature of matter. By showing that water was not an indivisible element, but a compound, Boyle laid the foundation for modern chemistry and revolutionized our understanding of the world around us. His legacy as a pioneering scientist and a champion of the scientific method continues to inspire generations of researchers to question, experiment, and seek the truth.
Latest Posts
Latest Posts
-
What Does The Hardy Weinberg Principle Relate To
Nov 16, 2025
-
The Author Of The Nature Of The Chemical Bond
Nov 16, 2025
-
Thyroid Removal Long Term Side Effects
Nov 16, 2025
-
What Is The Success Rate Of Radiation Therapy Stage 4
Nov 16, 2025
-
Single Molecule Mass Spectrometry Protein Us Patent Application
Nov 16, 2025
Related Post
Thank you for visiting our website which covers about Robert Boyle's Electrolysis Of Water Showed That _____. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.