Less Than 10 Cfu G Meaning
umccalltoaction
Dec 02, 2025 · 13 min read
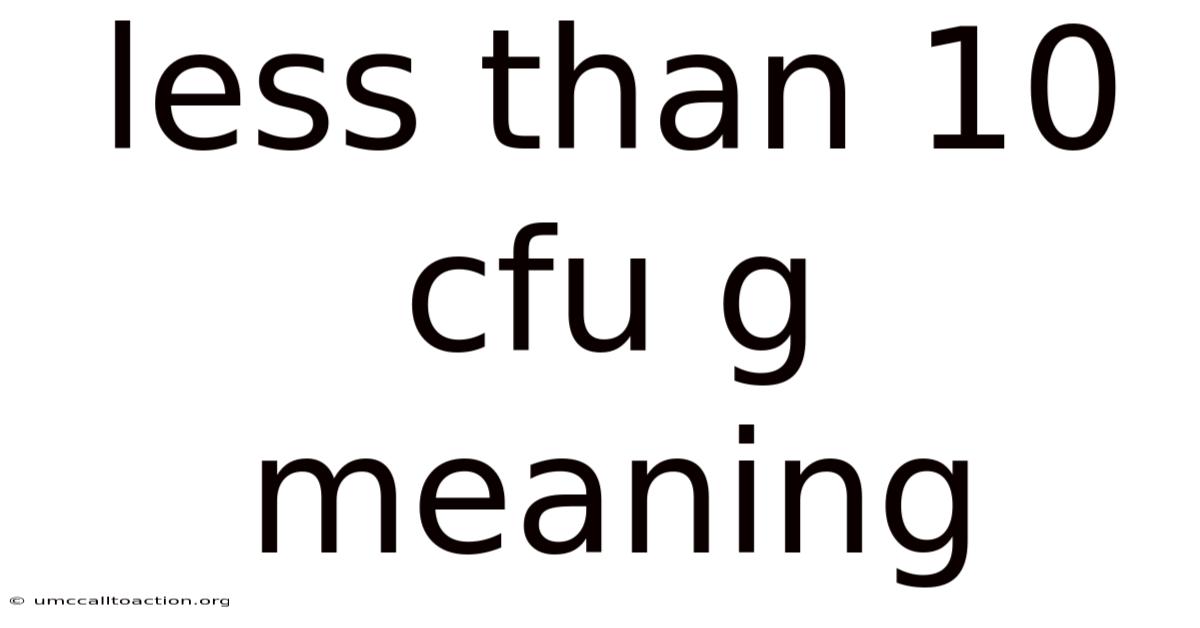
Table of Contents
Let's explore what "less than 10 CFU/g" signifies in the world of microbiology, touching upon its implications, the methods used to determine it, and why it's important across various industries.
Understanding CFU/g: A Microbial Yardstick
CFU/g stands for Colony Forming Units per gram. It's a fundamental unit of measurement in microbiology used to quantify the number of viable (living and able to multiply) bacteria or fungal cells present in a gram of a sample. Think of it as a microbial headcount, providing a standardized way to express the concentration of microorganisms in a given substance. A "low" CFU/g count, such as less than 10, indicates a relatively small number of viable microorganisms within that gram.
The concept hinges on the ability of a single viable microbial cell to multiply under suitable conditions, forming a visible colony on an agar plate. This colony is then counted as a single CFU, even though it originated from just one cell. CFU/g is essential because:
- It quantifies microbial contamination: It tells us how "dirty" a sample is, microbiologically speaking.
- It ensures product safety and quality: In food, pharmaceuticals, and cosmetics, low CFU/g counts are often crucial for consumer safety and product efficacy.
- It monitors sanitation and hygiene: Regular CFU/g testing helps assess the effectiveness of cleaning and disinfection procedures.
- It guides research and development: In biotechnology and environmental microbiology, CFU/g measurements are used to track microbial growth and activity.
Why Less Than 10 CFU/g Matters
The phrase "less than 10 CFU/g" represents a benchmark, a threshold of microbial cleanliness. Achieving this level is often the goal in several scenarios:
- Food Safety: Many food safety regulations specify maximum acceptable CFU/g levels for different types of food products. A level of less than 10 CFU/g is typically considered very good, indicating a low risk of spoilage or foodborne illness caused by that particular group of microorganisms being tested. However, it's crucial to remember that this applies to specific microorganisms. For example, a processed food might need to have less than 10 CFU/g of Salmonella or E. coli, but a higher count of beneficial bacteria might be acceptable or even desirable (as in the case of yogurt).
- Pharmaceuticals and Cosmetics: These industries demand stringent microbial control. Low CFU/g counts are vital to ensure product sterility (or, at least, a very low bioburden) and prevent infections in patients or consumers. Injectable drugs, eye drops, and products applied to damaged skin require particularly strict limits on microbial contamination. A product with "less than 10 CFU/g" is far less likely to cause adverse reactions or compromise the health of the user.
- Water Quality: While drinking water isn't usually assessed by CFU/g in the same way as solids, the principle applies. Regulations often specify limits on the number of coliform bacteria (indicator organisms) per milliliter, and a very low count is essential for safe consumption.
- Cleanroom Environments: In industries requiring ultra-clean conditions (electronics manufacturing, aerospace, advanced materials), air and surface monitoring for CFU/g helps maintain a sterile or near-sterile environment, preventing contamination of sensitive products or processes.
- Medical Devices: Medical devices, especially those that come into contact with sterile body tissues or fluids, must meet rigorous microbial limits. "Less than 10 CFU/g" might be a requirement for certain types of devices before sterilization.
Methods for Determining CFU/g
Determining CFU/g involves a series of microbiological techniques. Here's a general overview:
-
Sample Collection: Proper sample collection is critical. The sample must be representative of the entire batch or area being tested, and it must be collected aseptically to avoid introducing external contamination.
-
Sample Preparation: The sample usually needs to be diluted before plating.
- Solid Samples: A known weight (e.g., 1 gram) of the sample is mixed with a sterile diluent (e.g., sterile saline or peptone water) and homogenized. This creates a suspension of microorganisms.
- Liquid Samples: The liquid sample can be used directly or diluted as needed.
-
Serial Dilution: The initial suspension is subjected to a series of tenfold dilutions. For example, 1 ml of the suspension is added to 9 ml of sterile diluent, creating a 1:10 dilution. This process is repeated to create dilutions of 1:100, 1:1000, 1:10,000, and so on. Serial dilutions are necessary because the original sample may contain too many microorganisms to count accurately on a single plate.
-
Plating: A known volume (typically 0.1 ml or 1 ml) of each dilution is then plated onto an agar plate. Common plating methods include:
- Spread Plating: The diluted sample is spread evenly over the surface of the agar using a sterile spreader.
- Pour Plating: The diluted sample is mixed with molten agar, which is then poured into a sterile Petri dish and allowed to solidify.
The agar plates contain nutrients that support the growth of the target microorganisms. The choice of agar depends on the type of microorganisms being tested. For example, nutrient agar supports the growth of a wide range of bacteria, while Sabouraud dextrose agar is used for fungi. Selective agars are used to isolate specific types of microorganisms.
-
Incubation: The inoculated agar plates are incubated at a specific temperature (usually 30-37°C for bacteria and 25-30°C for fungi) for a specific period (usually 24-48 hours). During incubation, the viable microorganisms multiply and form visible colonies.
-
Counting Colonies: After incubation, the number of colonies on each plate is counted. Only plates with a countable number of colonies (typically between 30 and 300) are used for calculating CFU/g. Plates with too few colonies are statistically unreliable, while plates with too many colonies are difficult to count accurately due to overcrowding.
-
Calculation: The CFU/g is calculated using the following formula:
CFU/g = (Number of colonies x Dilution factor) / Volume platedFor example, if you plated 0.1 ml of a 1:1000 dilution and counted 50 colonies, the CFU/g would be:
CFU/g = (50 x 1000) / 0.1 = 500,000 CFU/gHowever, if no colonies are observed on the plate from the least diluted sample, you can only report the result as "less than" a certain value. For example, if you plated 1 ml of a 1:10 dilution (the least diluted) and saw no colonies, you can report the result as "less than 10 CFU/g." This is because if there were any colonies, they would have to number at least one. That single colony, adjusted for your dilution and plating volume, translates to 10 CFU/g.
Factors Affecting CFU/g Results
Several factors can influence CFU/g results:
- Sampling Technique: Improper sampling can lead to inaccurate results. The sample must be representative of the entire batch or area being tested, and it must be collected aseptically.
- Dilution Errors: Inaccurate dilutions can significantly affect the final CFU/g calculation.
- Plating Technique: Uneven spreading or pouring of the agar can lead to uneven distribution of microorganisms and inaccurate colony counts.
- Incubation Conditions: Temperature and incubation time must be carefully controlled to ensure optimal growth of the target microorganisms.
- Counting Errors: Inaccurate colony counts can arise from human error or from overcrowding on the plates. Automated colony counters can improve accuracy and efficiency.
- Microbial Physiology: The physiological state of the microorganisms can affect their ability to form colonies. Stressed or injured cells may not be able to grow on agar plates, leading to an underestimation of the total number of viable cells.
- Choice of Media: The type of agar used can affect the growth of different microorganisms. Using an inappropriate agar may lead to an underestimation of the total number of viable cells.
"Less Than 10 CFU/g" and the Limit of Detection
It's vital to understand that "less than 10 CFU/g" doesn't necessarily mean that the sample is completely sterile or free of microorganisms. It simply means that the number of viable microorganisms is below the limit of detection of the method used. The limit of detection depends on the sample volume plated, the dilution factor, and the lowest number of colonies that can be reliably counted.
For example, if you only plate 0.1 ml of the undiluted sample, the lowest detectable CFU/g would be 10 (if you saw one colony). If you need to detect lower levels of contamination, you would need to plate a larger volume or use a more sensitive method, such as enrichment cultures or molecular techniques.
Enrichment cultures involve incubating the sample in a nutrient-rich broth to allow any microorganisms present to multiply to detectable levels. Molecular techniques, such as PCR (Polymerase Chain Reaction), can detect even very small numbers of microorganisms by amplifying their DNA. However, PCR detects DNA, not necessarily viable organisms. A positive PCR result could indicate the presence of dead cells or free DNA, which would not be detected by CFU plating.
Statistical Considerations and Replicates
Microbial counts, including CFU/g measurements, are inherently variable. The distribution of microorganisms in a sample is rarely perfectly uniform, and the growth of colonies on agar plates can be affected by subtle variations in incubation conditions or media composition. Therefore, it's essential to perform replicate measurements and apply statistical analysis to obtain reliable results.
Typically, at least three replicate plates are prepared for each dilution. The CFU/g is then calculated for each replicate, and the mean and standard deviation are calculated. The standard deviation provides a measure of the variability of the results. A high standard deviation indicates that the results are highly variable and may not be reliable.
Statistical methods can also be used to determine the confidence interval for the CFU/g measurement. The confidence interval provides a range of values within which the true CFU/g is likely to fall, with a certain level of confidence (e.g., 95%).
Alternative Methods for Microbial Enumeration
While CFU/g plating is a widely used method, it has some limitations. It only detects microorganisms that can grow on the specific agar used, and it can be time-consuming and labor-intensive. Alternative methods for microbial enumeration include:
- Direct Microscopic Count: This method involves counting microorganisms directly under a microscope using a counting chamber. It is rapid and can detect both viable and non-viable cells, but it does not differentiate between different types of microorganisms, and it can be difficult to count small or clustered cells.
- Flow Cytometry: This method uses a laser beam and detectors to count and characterize individual cells in a fluid sample. It can rapidly and accurately count microorganisms and differentiate between different types of cells based on their size, shape, and fluorescence.
- Quantitative PCR (qPCR): As mentioned earlier, qPCR can detect and quantify specific microorganisms by amplifying their DNA. It is highly sensitive and specific, but it only detects the target microorganisms, and it does not necessarily indicate whether the cells are viable.
- ATP Bioluminescence: This method measures the amount of ATP (adenosine triphosphate), the energy currency of cells, in a sample. It provides a rapid and sensitive measure of the total number of viable cells, but it does not differentiate between different types of microorganisms.
- Impedance Microbiology: This method measures the electrical impedance (resistance to the flow of alternating current) of a microbial culture. As microorganisms grow, they metabolize nutrients and produce charged molecules, which changes the impedance of the culture. The change in impedance can be used to estimate the number of microorganisms present.
The choice of method depends on the specific application and the requirements for sensitivity, specificity, and speed.
Overcoming Challenges in Achieving "Less Than 10 CFU/g"
Achieving "less than 10 CFU/g" consistently can be challenging, especially in complex manufacturing environments. Here are some strategies for overcoming these challenges:
- Robust Cleaning and Disinfection Procedures: Implement and rigorously enforce effective cleaning and disinfection procedures for all surfaces, equipment, and personnel. Use validated disinfectants at the correct concentrations and contact times. Regularly monitor the effectiveness of cleaning and disinfection using swab tests or contact plates.
- Proper Hygiene Practices: Ensure that all personnel follow strict hygiene practices, including handwashing, wearing appropriate protective clothing (gloves, masks, gowns), and avoiding cross-contamination. Provide adequate training and reinforcement of hygiene protocols.
- Control of Air Quality: Maintain good air quality in manufacturing areas by using HEPA filters, controlling air flow, and minimizing dust and particulate matter. Regularly monitor air quality using air samplers.
- Water Quality Monitoring: If water is used in the manufacturing process, ensure that it meets the required microbial standards. Regularly monitor water quality and implement appropriate water treatment procedures (e.g., filtration, UV sterilization, chlorination).
- Raw Material Testing: Test raw materials for microbial contamination before use. Use suppliers who have robust quality control systems in place.
- Process Validation: Validate all manufacturing processes to ensure that they consistently produce products that meet the required microbial standards. This includes identifying critical control points and implementing appropriate monitoring and control measures.
- Environmental Monitoring: Implement a comprehensive environmental monitoring program to regularly assess the microbial quality of the manufacturing environment. This includes monitoring surfaces, air, and water.
- Continuous Improvement: Continuously review and improve cleaning, disinfection, and hygiene procedures based on environmental monitoring data and process validation results.
Real-World Examples
- Pharmaceutical Manufacturing: A pharmaceutical company manufacturing sterile injectable drugs must demonstrate that its manufacturing processes consistently achieve "less than 10 CFU/g" (or, more accurately, complete sterility) in the final product. This requires rigorous controls on raw materials, manufacturing environment, and sterilization processes.
- Food Processing: A food processing plant producing ready-to-eat salads must ensure that the salads have "less than 10 CFU/g" of Listeria monocytogenes, a bacterium that can cause serious foodborne illness. This requires careful washing and disinfection of the salad greens, as well as strict temperature control during storage and transportation.
- Cosmetics Production: A cosmetics manufacturer producing lotions and creams must ensure that its products have low levels of microbial contamination to prevent spoilage and skin infections. They might aim for "less than 10 CFU/g" of total aerobic bacteria and absence of specific pathogens like Pseudomonas aeruginosa.
- Hospital Environment: A hospital must maintain a clean and sanitary environment to prevent healthcare-associated infections. Regular monitoring of surfaces and equipment for CFU/g helps to assess the effectiveness of cleaning and disinfection procedures. While "less than 10 CFU/g" might be a target for certain high-risk areas (e.g., operating rooms), the specific limits would depend on the location and the type of microorganisms being monitored.
Conclusion
The requirement of "less than 10 CFU/g" is a powerful tool for ensuring product safety, quality, and efficacy across diverse industries. While achieving and verifying this standard demands meticulous attention to detail, robust methodologies, and a commitment to continuous improvement, the benefits are undeniable in protecting consumers, patients, and the integrity of sensitive processes. Understanding the principles behind CFU/g measurements, the limitations of the methods used, and the factors that can influence results is crucial for anyone working in microbiology, quality control, or regulatory affairs.
Latest Posts
Latest Posts
-
The Objective Of Layout Strategy Is To
Dec 02, 2025
-
Long Term Side Effects Of Proton Therapy
Dec 02, 2025
-
Control Reaches End Of Non Void Function
Dec 02, 2025
-
Which Of The Following Chemicals Do Not Directly Trigger Inflammation
Dec 02, 2025
-
8 7 Global Resistance To Established Power Structures
Dec 02, 2025
Related Post
Thank you for visiting our website which covers about Less Than 10 Cfu G Meaning . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.